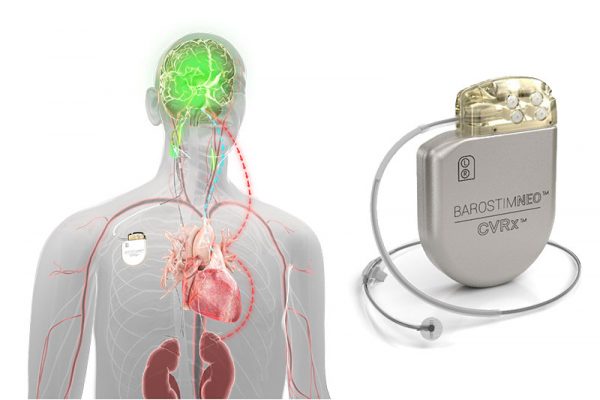
Web Barostim Baroreflex Activation Therapy BAT uses the power of the autonomic nervous system to improve symptoms of heart failure. Web Section 1886d of the Act sets forth a system of payment for the operating costs of acute care hospital inpatient stays under Medicare Part A Hospital Insurance based on prospectively set rates.

Fda Approves Device To Treat Patients With Heart Failure Wjar
Web Five-Star Quality Rating System.

. LTC Survey Manual Phase 3. Web BAROSTIM NEO System is indicated for the improvement of symptoms of heart failure quality of life six-minute hall walk and functional status for patients who remain symptomatic despite treatment with guideline-directed medical therapy are NYHA Class III or Class II who had a recent history of Class III have a left ventricular ejection. CVRx a commercial-stage medical device company focused on developing manufacturing and commercializing innovative.
Food and Drug Administration today approved the Barostim Neo System for the improvement of symptoms in patients with advanced heart failure who are not suited for treatment with other. Short-Mid-Term Fu of New COC36 patients. Section 1886g of the Act requires the Secretary to use a prospective payment system PPS to pay for the capital-related costs of inpatient.
Web BAROSTIM NEO System. Web The purpose of this study is to evaluate the investigational BAROSTIM NEO implantable device designed to electrically stimulate the baroreceptors located on one of the carotid arteries in theneck. DEPUY CERAMAX CERAMIC TOTAL HIP SYSTEM.
Patient stories Im able to do the things Ive always wanted to do-David Im back to walking three to four miles a day-Kevie Amazingly happy tears of joy I havent had in 10 years-Rhonda. Extended Phase BeAT-HF PAS. LTC Career Center.
This may result in improved heart function and reduced heart failure symptoms. SureScan Pacing System Post-Approval Study. Occupational Safety and Health Administration OSHA Workforce Career.
Web The metal device which is the size of a baked bean is inserted into one of the two carotid arteries which run on each side of the neck connecting the heart to the brain. Payroll Based Journal PBJ Mandatory Reporting. P070026 S004 DEPUY ORTHOPAEDICS INC.
Web 29072022 - MINNEAPOLIS July 29 2022 GLOBE NEWSWIRE - CVRx Inc. Quality AssurancePerformance Improvement QAPI Survey Preparedness.
About Us Cardiovascular Interventions

Barostim Neo Neuromodulation Implantable System Usa

Cvrx Barostim Neo Now Cleared For Mri Use In Europe Medaxs

A Barostim Neo Electrode Assembly And Implantable Pulse Generator Download Scientific Diagram

Investigational Device For Heart Failure Patients Stimulates Cells In Arteries To Improve Function Youtube

First Rheos And Second Barostim Neo Generation Baroreflex Download Scientific Diagram

Barostim Neo Electrical Stimulator Approved For Heart Failure In Europe Video Medgadget

Baroreflex Activation Therapy Barostim Neo System Tctmd Com

Barostim Neo Neuromodulation Implantable System Usa

Barostim Neo Electrical Stimulator Approved For Heart Failure In Europe Video Medgadget

Barostim Baroreflex Activation Therapy Cvrx
First Implant Made For Barostim Neo Device To Treat Hypertension Daic

A Barostim Neo Electrode Assembly And Implantable Pulse Generator Download Scientific Diagram
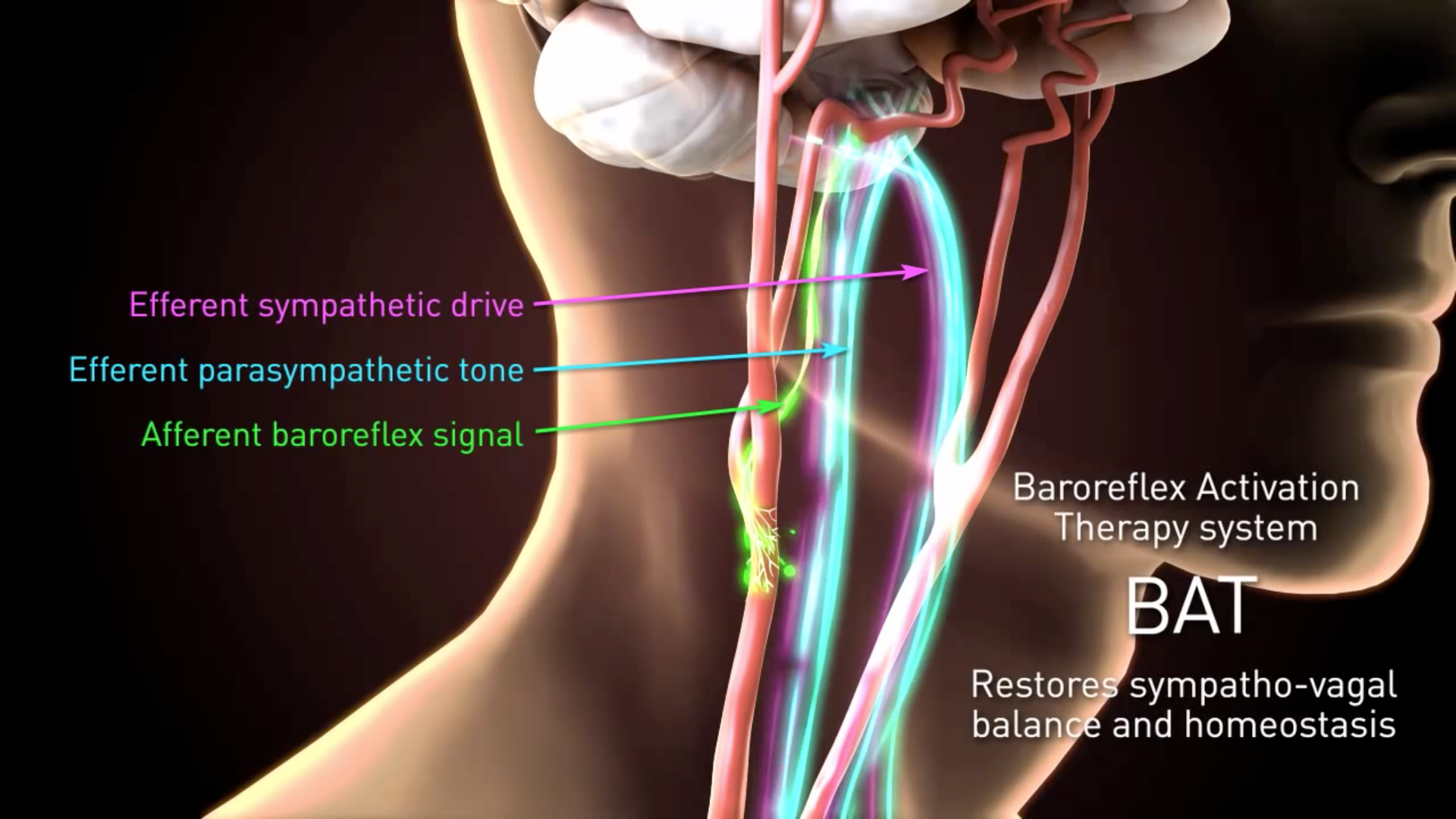
Barostim Neo 1 Radcliffe Cardiology
Cvrx S Barostim Neo Gets Ce Mark For Use With Mris Massdevice

Baroreflex Activation Therapy By The Rheos System And The Barostim Download Scientific Diagram


